To this end, we continuously train our employees, manufacture our medicines under state-of-the-art conditions, and not only check the production parameters during the manufacturing process, but also afterwards when we subject our products to chemical, physical and microbiological testing. We also pay attention to quality requirements during storage and transportation. All this to ensure that only high-quality medicines reach patients.
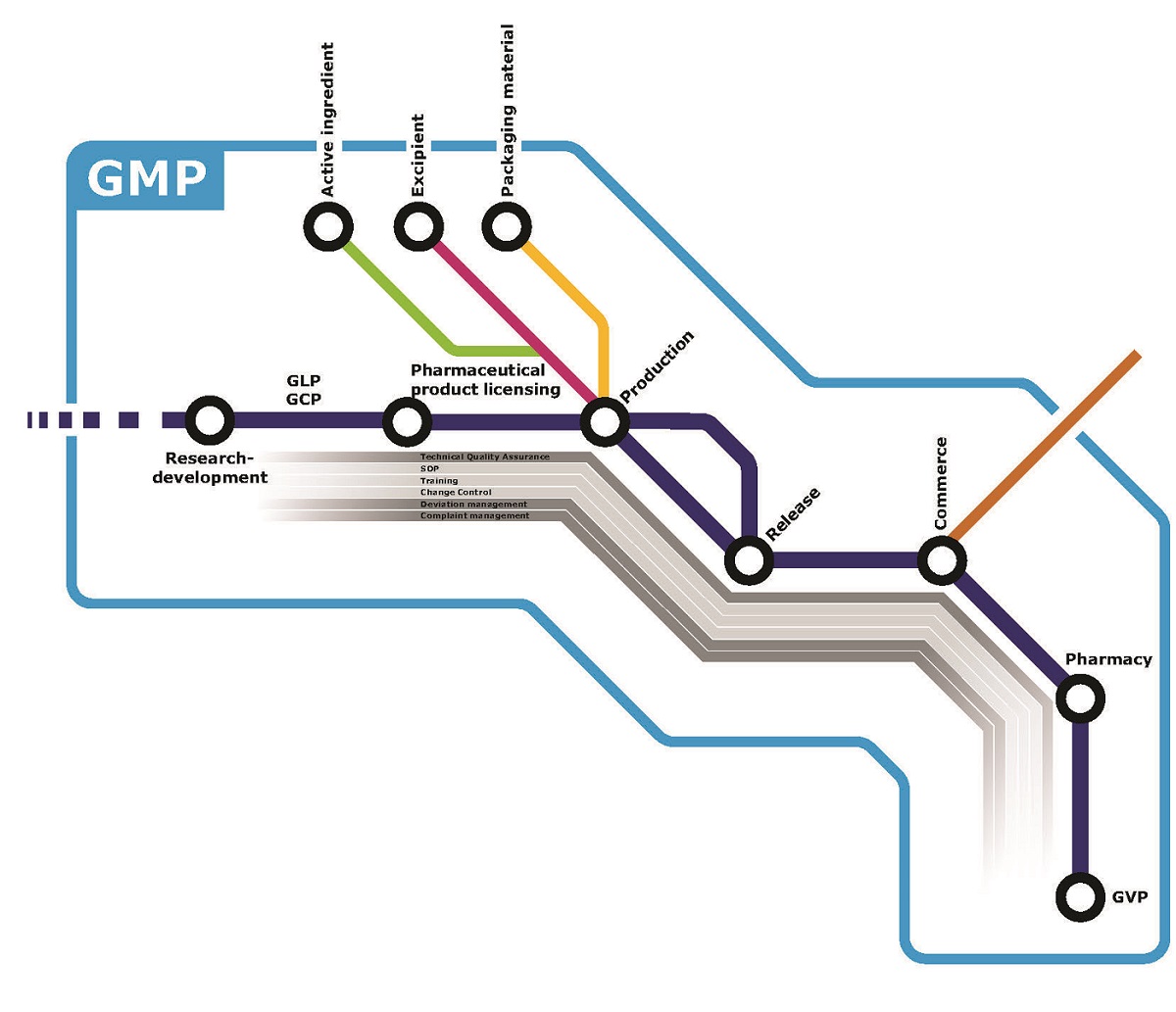
In manufacturing, we devote particular attention to compliance with the applicable technological and quality assurance regulations, as well as with domestic, European and other international laws and requirements.
We operate a comprehensive quality management system based on the requirements of the current GMP (Good Manufacturing Practice) guidelines, which includes risk management for the design, development and regulation of all products, devices and processes that may be a source of danger either for patients or for the Company. As we give priority to developing and harmonising the efficiency of the quality assurance system across the entire Group, we monitor the operation of our subsidiaries continuously and seek to develop the most consistent approach and procedures possible.
Our manufacturing activities and quality system at our parent company and production subsidiaries are regularly inspected by both our contractual partners and the authorities. In addition to the local authorities, in accordance with our trade relations, the Romanian, Polish, Russian, Belarusian, Yemeni, Chinese, Turkmen, Peruvian, South Korean, and Saudi authorities conducted inspections and examined the compliance of the production and quality management system. The adequacy of our quality system is recognized not only by the European authorities, but also by the FDA. We are very proud that for years these audits have concluded without critical remarks.