In our development projects, special emphasis is placed on providing state-of-the-art equipment and methods as well as employee expertise. The range of technological tools and equipment is constantly updated, as we consider it important to keep up with technological advances.
Employees participate in training programmes, presentations, courses and professional conferences, through which we aim to increase the level of professional competence of employees.
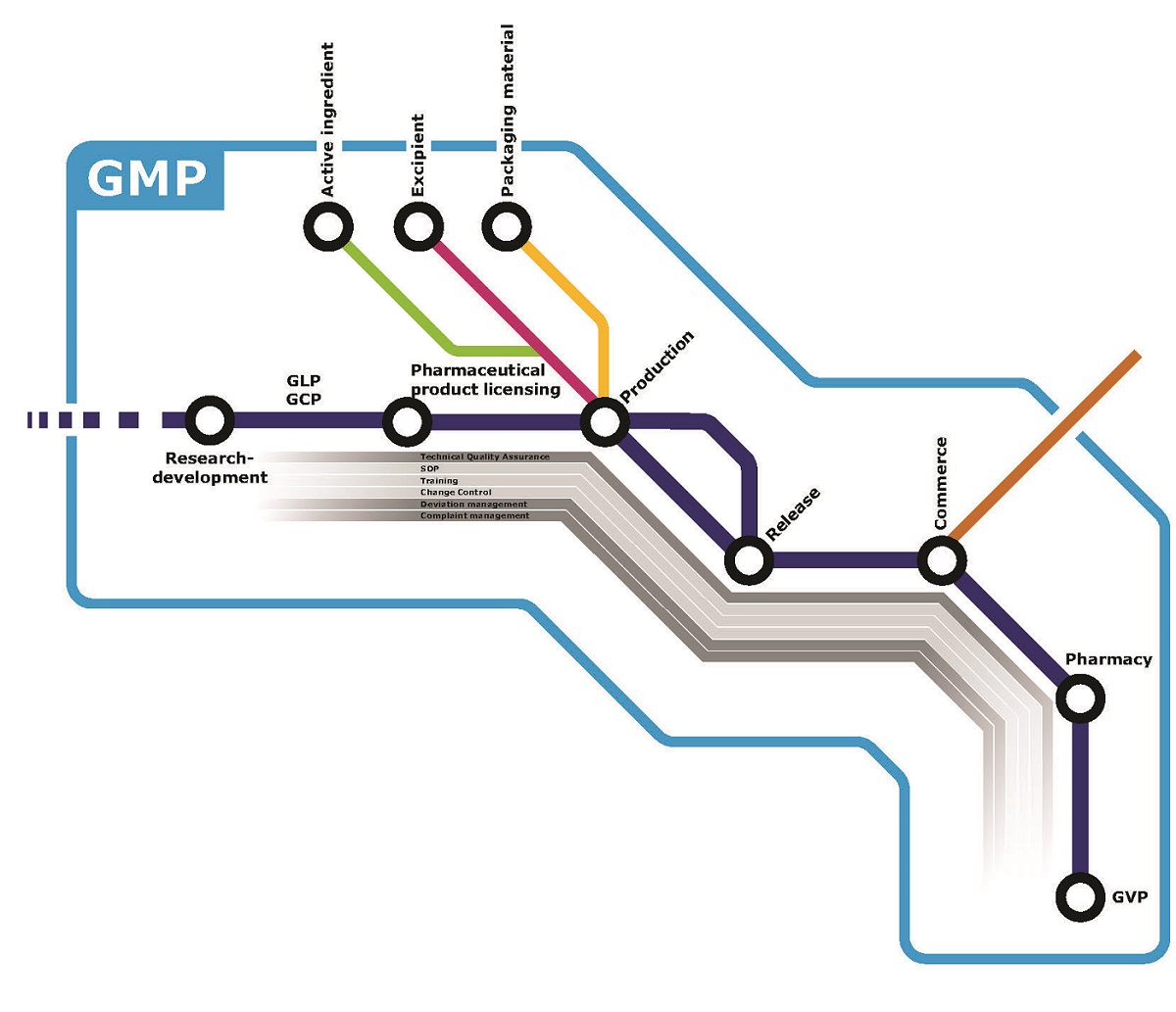
The company's pharmaceutical quality system encompasses all activities that can directly or indirectly influence product quality. Quality risk management is also implemented as a systematic process for assessing, controlling, communicating and re-examining risks to the quality of the medicine. Quality risk assessment is based on scientific knowledge and experience of the process and ultimately leads to patient protection. The level of effort, regulatory nature and documentation of the quality risk management process is commensurate with the level of risk.